Explore the Where Is The Most Energy Stored In An Atp Molecule article containing information you might be looking for, hopefully beneficial for you.
I recall my first encounter with ATP, the energy currency of cells, during my high school biology class. I was fascinated by how it could power so many crucial cellular processes. As I delved deeper into the topic, I discovered that the secret to understanding ATP’s energy-rich nature lies in its unique structure.

Where Is The Most Energy Stored In An Atp Molecule
Unveiling the Structure of ATP
ATP (adenosine triphosphate) consists of three components: adenine, a nitrogenous base; ribose, a five-carbon sugar; and a chain of three phosphate groups. These phosphate groups are linked together by phosphoanhydride bonds, which are high-energy bonds. When these bonds are broken, a significant amount of energy is released, making ATP a formidable energy source for the cell.
Breaking Down the Energy Stores
The most energy within an ATP molecule is stored in the second phosphate bond, referred to as the alpha-beta bond. This bond is particularly unstable, releasing a large amount of energy when broken. This energy is then accessible to fuel various cellular activities, such as muscle contraction, nerve impulse transmission, and chemical synthesis.
Understanding High-Energy Bonds
High-energy bonds are characterized by a negative ΔG (change in free energy), indicating that they are thermodynamically unstable. When a high-energy bond is hydrolyzed (broken down by water), the energy released can be harnessed to perform work. In the case of ATP, the hydrolysis of the alpha-beta phosphate bond releases approximately -7.3 kcal/mol of energy, which is a significant amount for biological reactions.
The Significance of ATP in Cellular Processes
ATP serves as the primary energy currency for all living organisms, providing the energy required for numerous cellular processes:
- Muscle Contraction: ATP hydrolysis powers the sliding of actin and myosin filaments in muscles, leading to muscle contraction.
- Active Transport: Cells use ATP to actively transport molecules against concentration gradients, essential for maintaining cellular homeostasis.
- Protein Synthesis: The energy released from ATP hydrolysis is utilized during protein synthesis, where amino acids are linked together to form polypeptide chains.
- Nerve Impulse Transmission: ATP fuels the propagation of nerve impulses along neurons, enabling communication within the nervous system.
- Chemical Synthesis: ATP provides the energy necessary for various chemical synthesis reactions, including the production of complex molecules like DNA and RNA.
Tips and Expert Advice for Understanding ATP
- Visualize the Structure: Create a mental picture of the ATP molecule, focusing on the arrangement of its components and the location of the high-energy phosphate bonds.
- Emphasize the Energy Release: Understand that the energy released upon hydrolysis of the alpha-beta phosphate bond is substantial and drives cellular processes.
- Connect to Biological Significance: Link the properties of ATP to its fundamental role in powering countless cellular functions.
Frequently Asked Questions
Q: Why is the second phosphate bond in ATP particularly high in energy?
A: The second phosphate group is attached to the alpha carbon of ribose, which destabilizes the bond due to steric hindrance. This instability leads to a negative ΔG and a greater release of energy upon hydrolysis.
Q: How is ATP regenerated after being hydrolyzed?
A: ATP can be regenerated through several mechanisms, including substrate-level phosphorylation, oxidative phosphorylation, and photophosphorylation. These processes add a phosphate group back to ADP (adenosine diphosphate) to reform ATP.
Q: What are the implications of ATP depletion in cells?
A: Depletion of ATP can lead to cellular dysfunction and even cell death. The cell’s ability to perform essential processes becomes compromised, impairing metabolism, communication, and growth.
Conclusion
The energy stored in an ATP molecule is primarily located in the second phosphate bond, the alpha-beta bond. This phosphoanhydride bond is highly unstable and releases a significant amount of energy when broken, providing the necessary power for numerous cellular processes. By understanding the structure and energy dynamics of ATP, we gain insights into the fundamental mechanisms that drive life.
Are you intrigued by the fascinating world of cellular energy? Continue your exploration with further readings and discussions on the vital role of ATP in biology.
Where Is The Most Energy Stored In An Atp Molecule
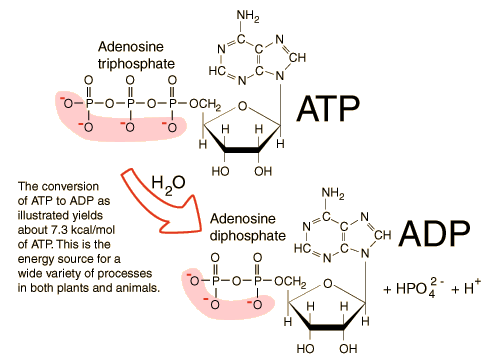
Image: socratic.org
Thank you for visiting our website and taking the time to read Where Is The Most Energy Stored In An Atp Molecule. We hope you find benefits from this article.







